BillerudKorsnäs secures medical paper production
What is BillerudKorsnäs doing to ensure timely deliveries of medical papers in the unique state of affairs of the Covid-19 pandemic? Now more than ever, BillerudKorsnäs has a item role to play in ensuring the stable production of medical papers for medical packaging across the globe.
The Covid-xix pandemic is affecting us all in ways that nosotros could take never foreseen. What BillerudKorsnäs can exercise and are doing in these difficult times is, besides to ensure the safest possible working surroundings for our employees, is to provide stable production of SteriKraft and MediKraft medical paper to our medical packaging customers all over the world.
In its mills in Skärblacka and Beetham, the company has prioritized production for medical newspaper and its overall logistic flow, and it is prioritizing deliveries of SteriKraft and MediKraft medical paper. In addition, BillerudKorsnäs has comprehensive contingency plans in place to mitigate whatsoever potential interruptions due to raw material, other resources, and workers. The company's cantankerous-functional geographical teams, together with sales, practise their utmost to go along maintain the highest service level in all regions.
Designed to continue devices sterile
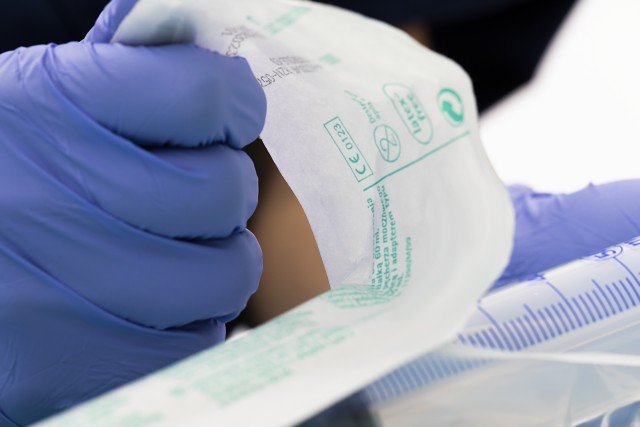
In the midst of the Covid-nineteen pandemic, a much more widespread understanding of the importance of sterile medical supplies and devices has emerged. Healthcare workers require a vast number of sterile medical devices such equally syringes, gloves, dressings, and other products. Medical packaging is essential in ensuring that devices are sterile and clean at the indicate of utilize.
The ability to keep medical devices sterile that is free from microorganisms from the time of sterilization until the betoken of utilize, is the single most important function of medical packaging. Medical packaging papers are used alongside many other materials, including packaging films, semi-rigid plastic trays, not-wovens, and other materials to form the sterile barrier systems needed to go on medical devices sterile.
The effective bulwark properties of medical papers are accomplished through their multilayer fiber structure, an constructive tortuous path of pores, and a well-balanced composition of newspaper properties. The tortuous path produces a uniform and consequent bacteriological and fluid bulwark to maintain sterility and foreclose microorganisms from reaching the sterile contents.
Ensuring the microbial barrier
The most common test for microbial barrier used in Europe is DIN 58953-6. During the test, a highly concentrated bacterial solution is dropped onto the tested cloth. Then on the reverse side, it is contacted with nutrition agar to check if whatsoever bacteria has come up through the paper.
Stringent requirements
Stringent requirements on medical packaging and its design entail very high demands on the materials used. The material must be appropriate for the intended use, uniform with the called sterilization method, and designed to maintain sterility. To ensure that the material used in the packaging is fit for purpose, it must comply with international standards – the two most important ones being ISO 11607 (office one) and EN 868 (parts two-ten).
ISO 11607 – specifies the requirements and exam methods for materials and packaging, which must be fulfilled. EN 868 parts 2 to 10 – specify demands on mechanical, concrete, and chemical parameters related to a specific type of packaging and packaging textile.
Rigorous testing
Before BillerudKorsnäs places any newspaper grades on the market to be used in medical packaging, a series of different tests are performed to ensure compliance with relevant standards. The newspaper is produced according to the product standard EN 868, which specifies high demands on mechanical, physical, and chemic parameters.
The corresponding paper parameters, such every bit tear and burst strength, porosity, and pore size, to proper noun a few, are continuously controlled in the paper mill, to make certain the medical packaging paper e'er complies with the standard. Before the paper is ready to be delivered to its customers (converters and industrial medical device manufacturers), additional testing is performed to ensure that the newspaper fulfills all requirements according to ISO 11607.
The above article based on a press release has been edited past Naresh Khanna
Source: https://packagingsouthasia.com/she-safety-health-and-environment/sustainability-health/billerudkorsnas-secures-medical-paper-production/
0 Response to "BillerudKorsnäs secures medical paper production"
Post a Comment